Example Problems for Entrance Examinations
Preliminary round
Problem 1.
Give an example of chemical reaction between a salt and an acid leading to the formation of another salt and a base.
(1 point) [Solution]
Problem 2.
Write the reactions involved in the following sequence of oxidation number transformations:
(2 points) [Solution]
P0  P-3
P-3  P-3
P-3  P+5
P+5  P+5
P+5
Problem 3.
Write the reactions of chromium (III) bromide with hydrogen peroxide in acidic and basic media.
(2 points) [Solution]
Problem 4.
Write the reactions involved in the following sequence:
C10H12  C3H6O2
C3H6O2  C3H5OCl
C3H5OCl  C10H12O2.
C10H12O2.
In the reactions, specify the structures of all substances.
(3 points) [Solution]
Problem 5.
Write the reactions involved in the following sequence of transformations:
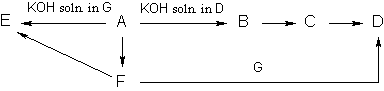
Determine the unknown substances.
(4 points) [Solution]
Problem 6.
Three substances A, B, and C were mixed in the amount of three moles each. When the equilibrium
A + B  2C
2C
was reached, five moles of substance C were found in the system. Calculate the equilibrium constant. Determine the equilibrium composition (in mol.%) of mixture, obtained by adding three moles of A and two moles of B to one mole of C at the same temperature.
(4 points) [Solution]
Problem 7.
Combustion of 10.7 g of unknown substance gave 30.8 g of CO2, 8.1 g of Н2O, and 1.4 g of N2. The complete hydrogenation of the same sample of this substance required 9.0 l of hydrogen at temperature 227 oC and pressure 138.5 kPa. The gas obtained by complete evaporation of 3.21 g of the substance occupied the volume of 1.25 l at temperature 227 oC and pressure 99.7 kPa. Draw the four possible structures of the unknown substance.
(4 points) [Solution]
See also:
- Example problems in chemistry for the entrance
exams (main round)
- Special Program developed
for chemistry entrance examinations in Moscow State University.
Chemistry Department of Moscow State University




